About the vantage study
The objective of this study is to evaluate if the investigational medication, volixibat, is a potential treatment for itch in PBC.
To participate in this clinical research study, you must meet a set of qualifying criteria. Some are listed here. A study representative will be able to give you more information to see if you or your loved one qualifies.



There are additional study requirements to participate.
A study representative will discuss them with you during the screening period.


The VANTAGE Study is seeking adults with PBC to learn if the investigational medication, volixibat, might be an effective and safe treatment option for itch due to PBC compared to placebo (drug with no active ingredient).


The study consists of a screening period, treatment period, and follow-up period.
The study will last at least 36 weeks (~8 months) with the option to participate in a Long-Term Extension (LTE) treatment period, in which participants are guaranteed to receive the investigational medication, volixibat.
*For USA only: You may be eligible to complete all visits from home.
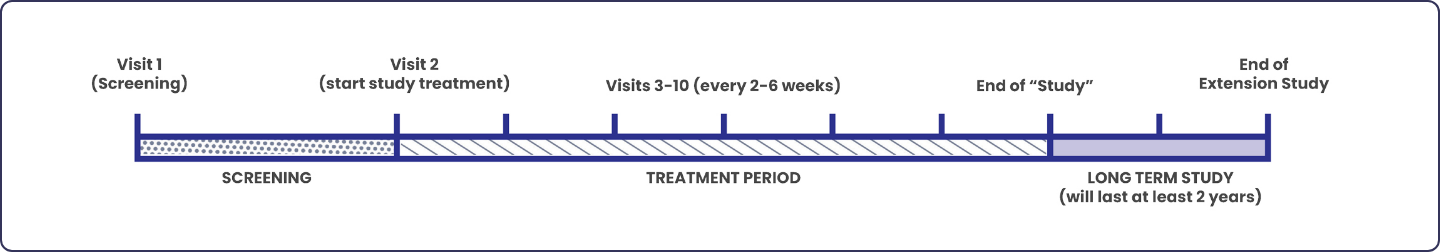
During the screening period, you will be evaluated to determine if you meet the criteria for entry into the study. Study staff will assess your health and determine if you may participate in the VANTAGE Study. You will be asked to record your itch score once every day. Recording your itch score will take less than 2 minutes.
During the treatment period, you will be randomly placed on volixibat or placebo, which you will take twice a day by mouth. The study team will perform study related tests and procedures and ask questions. Some visits can be performed virtually or remotely. You will be asked to continue recording your itch score once every day.
At the end of the treatment period, you will be given the option to continue to the Long-Term Extension (LTE) treatment period, where every patient, regardless of whether they were on volixibat or placebo during the treatment period, will be guaranteed to receive the investigational medication, volixibat, and be monitored by your study doctor for at least 2 years. Some visits can be performed virtually or remotely. Participants will be asked to continue recording their itch score once every day.

Primary billary cholangitis (PBC) is a progressive inflammatory liver disease that causes damage to the bile ducts inside the liver. It is considered an autoimmune disease and can lead to irreversible scarring of liver tissue and liver failure requiring transplantation. While PBC affects mostly women, men may also develop this condition.
There are currently no approved therapies to treat itch associated with PBC.


A clinical trial tests the safety and effectiveness of an investigational medication in human volunteers. Every investigational medication goes through the clinical trial process. Therefore, participants play a vital role in advancing medicine for present and future generations.
An investigational medication has not been approved by the U.S. Food and Drug Administration (FDA) or other regulating body but is allowed to be administered to people for research purposes.
A placebo has no active ingredient and is used to compare the effects of the investigational medication. The placebo will look like the investigational medication and will be administered the same way.
Agreeing to join a clinical trial is entirely up to you. Even if you sign the informed consent form, you’ll be free to leave the trial at any time.
If you agree to participate, you would be expected to follow the rules and instructions to the best of your ability. If you are not able to follow these rules and instructions, you may be asked to withdraw from the study.
In order to provide maximum protection for your health, the study will be under the direct supervision of the study doctor and will be conducted by trained personnel. You will need to provide all information about your current and past health (medical history) at the Screening Visit and at each Follow-Up Visit, including participation in any other research studies. This information is needed to protect your health.
If you have a primary doctor, it is strongly recommended that you inform him/her of your interest to participate in this research study.
Yes, for attending office visits unless specified by your study site. You may also be reimbursed for study-related expenses, such as travel for site visits. The study drug and all study-related office visits, procedures, and examinations are provided at no cost to participants.
Mirum Pharmaceuticals, Inc., based in Foster City, California, is a biopharmaceutical company focused on the development and commercialization of therapies to treat debilitating liver diseases. Mirum currently has two ileal bile acid transporter (IBAT) inhibitors; an investigational medication, volixibat for pediatric and adult liver diseases in development stage and one approved medication.
Mirum works closely with patient advocates and the patient communities to understand and address the most critical aspects affecting patients and families living with rare liver diseases.
For more information about Mirum, please visit MirumPharma.com.
More information on this clinical trial can be found by visiting clinicaltrials.gov and searching the study ID, NCT05050136 or VLX-601, and/or the study name, VANTAGE.

Clinical trials are important to advancing research and information from the study may help doctors learn more about PBC treatments that may potentially help other PBC patients in the future.

Thank you for your interest!